Abstract
Introduction: Bone marrow fibrosis (BMF) is a primary pathological and diagnostic feature of myelofibrosis (MF). BMF biopsies are evaluated and scored using updated WHO criteria, which consist of four escalating grades of severity: G0-G3 (Arber et al, 2016). Although BMF grade is not featured in the IPSS or DIPSS prognostic scoring systems, several studies have associated increasing degree of BMF with poor prognosis.
Current clinical research efforts in MF often assess BMF, with the ultimate intent of demonstrating disease modification. To date, limited clinical data have compared efficacy outcomes in MF such as survival or symptom, splenic, and/or anemia responses with BMF changes. Here, we present BMF data from over 300 JAK inhibitor (JAKi)-naïve patients who participated in the double-blind, randomized, Phase 3 SIMPLIFY-1 (S1) study of momelotinib (MMB), an inhibitor of JAK1, JAK2 and ACVR1, vs ruxolitinib (RUX), a JAK1 and JAK2 inhibitor.
Methods: BMF biopsies were collected pre-treatment (baseline), following 24 weeks of randomized treatment (RT) with either MMB or RUX, and at Week 96 during open-label treatment with MMB. Grading was performed by local hematopathologists and included assessments of reticulin and collagen. Other efficacy assessments included MFSAF symptom scoring, spleen volume imaging, transfusion independence (TI) status, and hemoglobin (Hgb) levels. Survival was estimated using KM analysis.
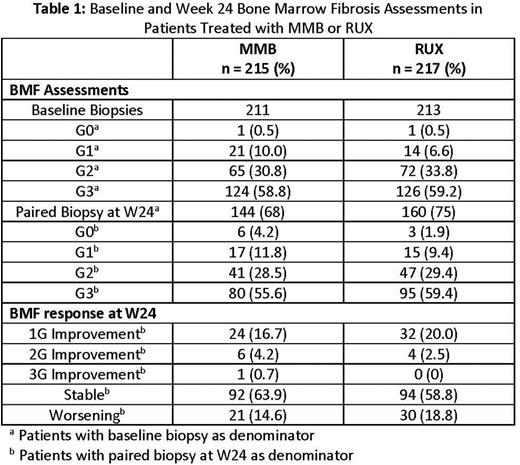
Results: In S1, 128 sites in N. America, Europe & APAC enrolled patients; 211/215 patients randomized to MMB and 213/217 patients randomized to RUX had baseline BMF assessments with 59% in each arm registering as G3. Of these patients, 144 and 160 on MMB and RUX, respectively, had a paired biopsy at Week 24 (W24). Of patients with a W24 paired biopsy, the proportion with stable or improved BMF was similar between treatment arms: 22% who received MMB had a ≥1G improvement compared with 23% of patients who received RUX, and 85% of patients had stable or improved BMF on MMB compared to 81% on RUX (Table 1).
In the MMB arm, 87% of the patients with BMF improvement of ≥1G were also W24 TI responders (TI-R), compared to 76% of those with stable or worsening BMF. In the RUX arm, 44% of those with ≥1G improvement in BMF and 56% with stable or worsening of BMF were also W24 TI-R. Furthermore, Hgb levels were noted to increase on MMB, regardless of BMF improvement or worsening, while Hgb levels were observed to decrease on RUX in patients with both improving and worsening BMF. No association between BMF outcome and spleen or symptom outcomes was noted for MMB or RUX.
In both treatment arms, no improvement in overall survival (OS) was observed for those achieving ≥1G BMF improvement (MMB HR=0.78, p=0.5203; RUX→MMB HR=1.27, p=0.4789). Although not statistically significant, those registering worsening of BMF grade trended to have improved OS compared to those with no change (MMB HR=0.570; RUX HR=0.597) or improvement (MMB HR=0.818; RUX HR=0.523).
Discussion: Improvement in BMF has been pursued as an endpoint in clinical trials and presumptively linked to disease modification. However, limited clinical data have been presented to date and the correlation of BMF changes with survival and other efficacy outcomes has not been well described.
Data presented here show that with both MMB and RUX approximately 20% of patients had ≥1G BMF improvement, whilst BMF was stable in >60%. Interestingly, for patients treated with MMB, Hgb levels increased and TI-R was achieved regardless of BMF changes, suggesting the previously described anemia benefit of MMB is a feature of its JAK1, JAK2, and ACVR1-mediated mechanism of action which is not reciprocated by RUX. Patients treated with RUX - even those achieving ≥2G BMF improvement - did not have improved Hgb levels, nor symptom or splenic response. Furthermore, long-term follow up of patients achieving ≥1G BMF improvement did not demonstrate OS advantage.
Conclusion: These data represent the most extensive analysis to date of the correlation of BMF changes with other outcome measures in JAKi-naïve patients with MF. Of particular note, anemia improvement was not linked with BMF changes. These findings bring into question the use of BMF assessment at W24 as a surrogate for clinical benefit. The clinical significance of BMF changes needs to be carefully evaluated to better understand its role in disease modification, the pathogenesis of MF, and clinically important patient outcomes.
Disclosures
Oh:Kartos: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sierra Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees; PharmaEssentia: Consultancy, Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Geron: Consultancy, Membership on an entity's Board of Directors or advisory committees; Disc Medicine: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgne/BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees; CTI BioPharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Constellation: Consultancy, Membership on an entity's Board of Directors or advisory committees; Blueprint Medicines: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees. Verstovsek:Novartis: Consultancy, Research Funding; Sierra Oncology: Consultancy, Research Funding; Incyte: Consultancy, Research Funding; NS Pharma: Research Funding; PharmaEssentia: Research Funding; ItalPharma: Research Funding; Promedior: Research Funding; Protagonist Therapeutics: Research Funding; Celgene: Consultancy, Research Funding; Gilead: Research Funding; Roche: Research Funding; Genentech: Research Funding; CTI BioPharma Corp.: Research Funding; Blueprints Medicines Corp.: Research Funding; AstraZeneca: Research Funding; Constellation Pharmaceuticals: Consultancy; Pragmatist: Consultancy. Gotlib:Incyte: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Deciphera: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; PharmaEssentia: Honoraria, Membership on an entity's Board of Directors or advisory committees; Blueprint Medicines Corporation: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Kartos: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Research Funding; Abbvie: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Cogent Biosciences: Consultancy, Research Funding; Allakos: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Gupta:Roche: Other: Participation on a Data Safety or Advisory board; Constellation Pharmaceuticals, Inc., a MorphoSys Company: Consultancy, Honoraria; Pfizer: Consultancy, Other: Participation on a Data Safety or Advisory board; Sierra Oncology: Consultancy; AbbVie: Consultancy, Other: Participation on a Data Safety or Advisory board; BMS Celgene: Consultancy, Honoraria, Other: Participation on a Data Safety or Advisory board; Novartis: Consultancy, Honoraria. Platzbecker:BMS/Celgene: Honoraria; Geron: Honoraria; Janssen: Honoraria; Silence Therapeutics: Honoraria; Takeda: Honoraria; Novartis: Honoraria; Abbvie: Honoraria; Jazz: Honoraria. Gisslinger:AOP Orphan Pharmaceuticals GmbH: Other: Grants and personal fees ; Novartis: Other: Grants and personal fees ; PharmaEssentia: Other: Personal fees ; BMS: Other: Personal fees. Devos:Morphosys: Consultancy; Alexion: Honoraria, Speakers Bureau; BMS/Celgene: Honoraria, Speakers Bureau; Novartis: Consultancy; Incyte: Consultancy. Kiladjian:AbbVie: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees; AOP Orphan: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees. McLornan:ABBVIE: Speakers Bureau; CELGENE BMS: Research Funding, Speakers Bureau; JAZZ: Honoraria, Speakers Bureau; NOVARTIS: Honoraria, Research Funding, Speakers Bureau. Perkins:Sierra Oncology: Honoraria, Other: Support for present manuscript; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria; Celgene: Other: Support for meetings and/or travel. Fox:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sierra Oncology: Honoraria, Membership on an entity's Board of Directors or advisory committees. McMullin:CTI: Consultancy; Sierra Oncology: Consultancy; Incyte: Consultancy, Speakers Bureau; Abbvie: Consultancy, Speakers Bureau; BMS: Consultancy, Research Funding; AOP: Research Funding, Speakers Bureau; Pfizer: Speakers Bureau; Novartis: Consultancy, Speakers Bureau. Mead:AbbVie: Consultancy, Speakers Bureau; Celgene/BMS: Consultancy, Research Funding, Speakers Bureau; Sierra Oncology: Consultancy, Speakers Bureau; CTI: Consultancy, Speakers Bureau; Pfizer: Consultancy, Speakers Bureau; Galecto: Consultancy, Research Funding, Speakers Bureau; Incyte: Consultancy, Speakers Bureau; Sensyn: Consultancy, Speakers Bureau; Karyopharm: Consultancy, Speakers Bureau; Alethiomics Ltd: Consultancy, Current equity holder in private company, Other: Co-founder and equity holder, Research Funding; Gilead: Consultancy, Speakers Bureau; Novartis: Consultancy, Honoraria, Research Funding, Speakers Bureau. Mayer:Sierra Oncology: Research Funding; Celgene: Research Funding. Sacha:Novartis: Consultancy, Honoraria, Speakers Bureau; Bristol Myers Squibb: Consultancy, Honoraria, Speakers Bureau; Incyte: Consultancy, Honoraria, Speakers Bureau; Pfizer: Consultancy, Honoraria, Speakers Bureau; Adamed: Consultancy, Honoraria; Angelini: Honoraria, Membership on an entity's Board of Directors or advisory committees; AOP Orphan: Honoraria, Speakers Bureau; BMs-Celgene: Honoraria, Speakers Bureau. Kawashima:Sierra Oncology: Current Employment. Huang:Sierra Oncology: Current Employment. Strouse:Sierra Oncology: Current Employment. Mesa:Promedior: Research Funding; AbbVie: Research Funding; Samus: Consultancy, Research Funding; Genotech: Research Funding; Geron: Consultancy; Roche: Consultancy; AOP: Consultancy; Bristol Myers Squibb: Consultancy; Blueprint: Consultancy; Incyte: Consultancy, Research Funding; Novartis: Consultancy; LaJolla Pharmaceutical: Consultancy; Constellation Pharmaceuticals, Inc., a MorphoSys Company: Consultancy, Research Funding; Celgene: Research Funding; CTI: Research Funding; Gilead: Research Funding; Sierra Oncology: Consultancy, Research Funding; Imago: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal